Abstract
Intravenous dexmedetomidine (Precedex®) provides both effective sedation in mechanically ventilated patients in an intensive care setting and effective procedural sedation. In these patient populations, it reduces the need for rescue sedation with intravenous propofol or intravenous midazolam and reduces opioid requirements. In addition, patients receiving dexmedetomidine are calm and easy to arouse and manage. Intravenous dexmedetomidine is generally well tolerated and is not associated with respiratory depression. Although the utilization of dexmedetomidine is associated with hypotension and bradycardia, both usually resolve without intervention.
Similar content being viewed by others
What is the rationale for developing the drug?
Sedation comprises a continuum represented by four progressive stages: minimal sedation, moderate sedation, deep sedation and general anaesthesia.[1] Patients in an intensive care setting commonly require sedation in order to improve patient comfort by relieving pain and anxiety, treat agitation, ameliorate the stress response and optimize mechanical ventilation.[2,3] The desired goal of sedation in these settings is usually a calm patient who can be easily aroused.[4]
Thus, intravenous sedation is considered integral for patients in an intensive care setting, particularly in those requiring mechanical ventilation, with its utilization for interventional procedures becoming more widespread.[4–6] A variety of agents are used to achieve sedation, including benzodiazepines (e.g. midazolam), general anaesthetics (e.g. propofol), opioids and α2-adrenergic receptor agonists such as dexmedetomidine (Precedex®) [table I].
How does the drug work?
Dexmedetomidine is a selective α2-adrenergic receptor agonist with a broad range of pharmacological properties.[7,8] Its sympatholytic effects are the result of decreased noradrenaline (norepinephrine) release in sympathetic nerve endings. The sedative effects of dexmedetomidine are thought to be mediated through decreased firing of the locus coeruleus, the major site of noradrenergic innervation in the brainstem.[7]
For whom is the drug indicated?
Table II provides a summary of the US prescribing information[9] for dexmedetomidine in the sedation of initially intubated and mechanically ventilated adult patients in an intensive care setting and in non-intubated adult patients prior to and/or during surgical and other procedures.
Prescribing summary of intravenous dexmedetomidine (Precedex®) for adult patients requiring short-term sedation in the US.[9] Consult local prescribing information for further details
What is its therapeutic efficacy in ventilated, intensive care patients?
The efficacy of intravenous dexmedetomidine as a short-term sedative for post-surgical patients in an intensive care setting was evaluated in two randomized, double-blind, placebo-controlled, multinational trials.[10–12] Eligible patients had undergone a surgical procedure (cardiac surgery, head and neck surgery, laparotomy or other) that was expected to require a minimum of 6 hours of post-surgical assisted ventilation.[10–12] The study drug was started within 1 hour of entry to the intensive care unit and continued for ≥6 hours of assisted ventilation, through weaning and extubation, and for ≥6 hours post-extubation, with a total treatment duration of up to 24 hours.[10–12]
Intravenous dexmedetomidine was an effective short-term sedative in this setting. The mean total dose of rescue sedation (intravenous propofol[10] or midazolam[11,12]) required to achieve and/or maintain a Ramsay Sedation Scale (RSS) score of ≥3 during the assisted ventilation period was significantly lower with dexmedetomidine than with placebo (table III). Moreover, significantly more dexmedetomidine than placebo recipients acquired and/or maintained a RSS score of ≥3 without rescue sedation with intravenous propofol[10] or midazolam[9,11,12] (table III).
Efficacy of intravenous dexmedetomidine vs placebo as a short-term sedative for post-surgical patients in an intensive care setting. Results of two randomized, double-blind, multinational trials[10–12] with supplemental data from the US manufacturer’s prescribing information.[9] Efficacy endpoints relate to the utilization of rescue sedation with propofol or midazolam,a with results reported in the intent-to-treat population for the assisted ventilation period and across the entire study drug administration period
The mean rate of rescue sedation with intravenous propofol required to achieve and/or maintain a RSS score of ≥3 during the assisted ventilation period was significantly lower with dexmedetomidine than with placebo (table III).[10] Moreover, the mean total dose[10] and/or mean rate[10,12] of rescue sedation with intravenous propofol[10] or midazolam[12] required over the entire study drug administration period was significantly lower with dexmedetomidine than with placebo (table III).
In addition, dexmedetomidine recipients required significantly (p < 0.05) less additional pain medication (morphine; assessed as mean total dose) than placebo recipients during the assisted ventilation period and the period from extubation to the end of the administration of the study medication.[10–12]
Mean Patient Management Index scores were also significantly (p < 0.05) lower with dexmedetomidine than with placebo, with lower scores corresponding to greater apparent calm, ease of communication (i.e. easier to arouse to answer questions or respond to neurological tests) and overall manageability of care, and greater tolerance of the endotracheal tube, the ventilator and the intensive care setting.[10–12]
Both dexmedetomidine and propofol provided effective sedation in patients who had undergone extensive cervical spine surgery and required overnight intubation and mechanical ventilation in a small, randomized, non-blind, single-centre study.[13] Patients received loading doses of dexmedetomidine 0.1 μg/kg/min (n = 16) or propofol 0.1 mg/kg/min (n = 16) over 10 minutes, followed by infusions of dexmedetomidine 0.4 μg/kg/h or propofol 1 mg/kg/h adjusted to maintain a RSS score of 2–4. All patients in both treatment groups achieved adequate RSS scores and had an adequate pain status and adequate movement in response to verbal commands.[13]
What is its therapeutic efficacy during procedural sedation?
Intravenous dexmedetomidine was effective as a primary sedative in adult patients undergoing awake fibre-optic intubation (AFOI)[14] or a variety of diagnostic or surgical procedures requiring monitored anaesthesia care (MAC),[15] according to the results of two pivotal, randomized, double-blind, multicentre trials. In the AFOI study,[14] significantly fewer dexmedetomidine than placebo recipients required rescue sedation with intravenous midazolam to achieve and/or maintain a RSS score of ≥2 during AFOI (table IV). In the MAC study,[15] rescue sedation with intravenous midazolam was not required to achieve and/or maintain an OAA/S score of ≤4 during MAC by significantly more dexmedetomidine than placebo recipients (table IV).[15]
Efficacy of sedation with intravenous dexmedetomidine in patients undergoing awake fibre-optic intubation[14] or a variety of diagnostic or surgical procedures requiring monitored anaesthesia care.[15] Results of two randomized, double-blind, placebo-controlled, multicentre studies; analyses are for the modified intent-to-treat population
The mean total midazolam dose was significantly lower in patients receiving dexmedetomidine than in those receiving placebo in both studies (table IV).[14,15] In addition, in the MAC study, the median time to midazolam administration was significantly longer with dexmedetomidine than with placebo, and significantly fewer dexmedetomidine than placebo recipients required further sedation (table IV).[15]
Small, randomized, controlled trials demonstrated that dexmedetomidine was generally associated with better outcomes than propofol[16] or propofol plus alfentanil[17] in patients requiring procedural sedation. For example, in patients with obstructive sleep apnoea syndrome who were undergoing a coblation-assisted upper airway procedure (n = 60), visual analogue scale scores assessing pain, how well the procedure was tolerated and satisfaction with the procedure significantly favoured dexmedetomidine versus propofol recipients at most timepoints, in a randomized, single-centre study (figure 1). In addition, significantly fewer dexmedetomidine than propofol recipients required supplemental analgesia (3% vs 23% of patients; p < 0.05).[16]
Efficacy of intravenous dexmedetomidine vs propofol for procedural sedation. Results of a randomized, single-centre study in which patients undergoing a coblation-assisted upper airway procedure received dexmedetomidine (1 μg/kg loading dose over 10 min followed by 0.7 μg/kg/h adjusted to maintain a Ramsay Sedation Scale score of 2–3) [n = 30] or propofol (n = 30).[16] Shown are patient-assessed visual analogue scales scores for pain, how well the procedure was tolerated and satisfaction with the procedure; scores ranged from 0 to 100 mm, with lower scores indicating better outcomes. Outcomes were assessed following radiofrequency volume tissue reduction of the soft palate (T1), oropharyngeal region (T2), tongue (T3) and inferior turbinate (T4). * p < 0.05, ** p < 0.01 vs propofol.
In addition, the mean postoperative Iowa satisfaction with anaesthesia scale score (primary endpoint) was significantly higher (indicating greater patient satisfaction) with intravenous dexmedetomidine than with intravenous propofol plus alfentanil in patients undergoing outpatient cataract surgery under MAC (50.3 vs 42.7; p < 0.001).[17] In this randomized, crossover study, 15 patients received dexmedetomidine 0.6 μg/kg/h and 16 patients received propofol 2 mg/kg/h plus alfentanil 20 μg/kg/h (both regimens were titrated to maintain a RSS score of 3) for the operation on the first eye, with patients switching treatments for the operation on the second eye; an interval of 1–2 weeks separated the operations.[17]
In another randomized, double-blind study, patients undergoing diagnostic flexible bronchoscopy received dexmedetomidine (bolus dose of 0.2 μg/kg followed by an infusion of 0.4–2 μg/kg/h) [n = 35] or remifentanil (bolus dose of 0.5 μg/kg, followed by an infusion of 1–5 μg/kg/h) [n = 35].[18] All patients received a bolus dose of propofol 0.5 mg/kg prior to study drug infusion.[18] Dexmedetomidine plus propofol recipients were significantly less likely than remifentanil plus propofol recipients to experience oxygen desaturation (primary endpoint) [3% vs 29% of patients; p = 0.01] or require oral suction (11% vs 37%; p = 0.03).[18] However, dexmedetomidine plus propofol recipients had significantly longer recovery times, required topical anaesthesia more frequently, and had poorer bronchoscopist cough and satisfaction scores than remifentanil plus propofol recipients (all p < 0.01).[18] There were no significant between-group differences with regard to the level of sedation, oxygen saturation during flexible bronchoscopy, patient cough and satisfaction scores, and the proportion of patients who were willing to undergo repeat bronchoscopy.[18]
What is its tolerability profile …
Intravenous dexmedetomidine was generally well tolerated when used for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation, with hypotension and/or bradycardia being the most commonly occurring adverse events.[9,10,14,15]
… versus placebo …
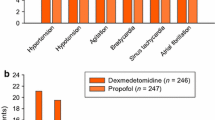
In postsurgical patients who were mechanically ventilated in an intensive care setting, sedation with dexmedetomidine was associated with a significantly (p < 0.005) higher incidence of hypotension (30% vs 10%) and bradycardia (9% vs 2%) and a significantly (p < 0.05) lower incidence of hypertension (12% vs 23%), atelectasis (0.5% vs 5%) and rigours (0.5% vs 4%) than sedation with placebo.[10] Hypotension and bradycardia tended to occur during or shortly after infusion of the dexmedetomidine loading dose. Hypotension generally resolved either without treatment or with changes in positioning and/or fluids, and bradycardia resolved either spontaneously or with medication (e.g. atropine). Dexmedetomidine did not appear to affect respiratory rate or oxygen saturation.[10]
Similarly, hypotension was the most commonly occurring adverse event in patients receiving dexmedetomidine who underwent procedural sedation.[14,15] For example, in the AFOI study, the incidence of hypotension was significantly higher (27.3% vs 6.0%; p = 0.0042) and that of tachycardia significantly lower (7.3% vs 24.0%; p = 0.0277) in dexmedetomidine than placebo recipients, with no significant difference between dexmedetomidine and placebo recipients in the incidence of bradycardia (7.3% vs 0%) or hypertension (23.6% vs 28.0%).[14] During the infusion period of the MAC study, the incidence of respiratory depression was significantly (p = 0.018) lower with dexmedetomidine loading doses of 0.5 or 1 μg/kg than with placebo (3.7% and 2.3% vs 12.7%).[15]
During infusion of the study drug in the AFOI study, there was no significant difference between dexmedetomidine and placebo recipients in the proportion of patients requiring intravenous fluids or medication to treat blood pressure or heart rate changes (12.7% vs 8.0%).[14] The incidence of intervention (study drug titration, intravenous fluid boluses or pharmacological therapy) for bradycardia, hypertension or tachycardia during study drug infusion in the MAC study did not significantly differ between patients receiving a loading dose of dexmedetomidine 0.5 or 1 μg/kg and those receiving placebo. However, the proportion of patients requiring intervention for hypotension was significantly higher in dexmedetomidine 0.5 μg/kg recipients than in placebo recipients (11.9% vs 3.2%; p = 0.046).[15]
… or active comparators?
Dexmedetomidine recipients had a significantly (p < 0.05) lower median heart rate than propofol recipients for up to 6 hours after administration of the loading dose in patients requiring overnight intubation and mechanical ventilation following extensive cervical spine surgery.[13] The median dopamine dose was significantly (p < 0.05) higher in dexmedetomidine than propofol recipients at 2 hours, but not at any other timepoint. In addition, atropine use was significantly (p = 0.04) higher with dexmedetomidine than with propofol.[13]
However, dexmedetomidine was better tolerated than propofol in patients requiring procedural sedation during a coblation-assisted upper airway procedure.[16] Propofol recipients were significantly (p < 0.05) more likely than dexmedetomidine recipients to experience airway obstruction or hypoxia, or to require esmolol for temporary haemodynamic support.[16]
Significantly fewer patients experienced hypertension with dexmedetomidine than with propofol plus alfentanil during cataract surgery (3.2% vs 25.8%; p < 0.05).[17] Systolic blood pressure was significantly (p < 0.05) lower in dexmedetomidine recipients than in propofol plus alfentanil recipients, with one case each of hypotension and bradycardia occurring with dexmedetomidine.[17]
Among patients undergoing diagnostic flexible bronchoscopy, there were no significant differences between dexmedetomidine plus propofol and remifentanil plus propofol in mean arterial pressure or heart rate, apart from a significantly (p < 0.05) higher heart rate with remifentanil plus propofol than with dexmedetomidine plus propofol at the time the bronchoscope was passed through the vocal cords.[18]
What is its current positioning?
Intravenous dexmedetomidine provides both effective sedation in mechanically ventilated patients in an intensive care setting and effective procedural sedation, and is generally well tolerated. In these patient populations, it reduces the need for rescue sedation with intravenous propofol or intravenous midazolam and reduces opioid requirements. Sedation with dexmedetomidine is also effective in terms of patient management, with dexmedetomidine recipients being calm and easy to arouse and manage. Dexmedetomidine is not associated with respiratory depression, and although hypotension and bradycardia may occur, both usually resolve without intervention. Thus, intravenous dexmedetomidine provides a further option as a short-term (<24 hours) primary sedative in mechanically ventilated adult patients in an intensive care setting and in non-intubated adult patients requiring procedural sedation.
Although dexmedetomidine is only indicated in the US for infusions of <24 hours duration, studies have also demonstrated the efficacy of dexmedetomidine when used for longer-term sedation in mechanically ventilated patients in the intensive care setting.[19] Moreover, a number of studies have examined the use of dexmedetomidine in paediatric patients, and as an adjunct to anaesthesia during surgery.[8]
References
Green SM, Mason KP. Reformulation of the sedation continuum. JAMA 2010 Mar 3; 303 (9): 876–7
Brown Lovato LM, Parker D. Procedural sedation in the acute care setting. Am Fam Physician 2005 Jan 1; 71 (1): 85–90
Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002 Apr; 96 (4): 1004–17
Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002 Jan; 30 (1): 119–41
Wunsch H, Kahn JM, Kramer AA, et al. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med 2009 Dec 1; 37 (12): 3031–9
Gan TG. Pharmacokinetic and pharmacodynamic characteristics of medications used for moderate sedation. Clin Pharmacokinet 2006; 45 (9): 855–69
Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011; 71 (11): 1481–501
Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 2012 Jan–Feb; 62 (1): 118–33
Precedex (dexmedetomidine hydrochloride) injection: US prescribing information. Lake Forest (IL): Hospira, Inc., 2010 Sep
Martin E, Ramsay G, Mantz J, et al. The role of the α2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med 2003 Jan-Feb; 18 (1): 29–41
FDA Center for Drug Evaluation and Research. Precedex (dexmedetomidine hcl injection); medical review(s). Part 1 [online]. Available from URL: http://www.accessdata.fda.gov [Accessed 2012 Jun 1]
US Food and Drug Administration Center for Drug Evaluation and Research. Precedex (dexmedetomidine hcl injection); medical review(s). Part 2 [online]. Available from URL: http://www.accessdata.fda.gov [Accessed 2012 Jun 1]
Terao Y, Ichinomiya T, Higashijima U, et al. Comparison between propofol and dexmedetomidine in postoperative sedation after extensive cervical spine surgery. J Anesth 2012 Apr; 26 (2): 179–86
Bergese SD, Candiotti KA, Bokesch PM, et al. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther 2010 Nov–Dec; 17 (6): 586–95
Candiotti KA, Bergese SD, Bokesch PM, et al. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg 2010 Jan; 110 (1): 47–56
Ma XX, Fang XM, Hou TN. Comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during coblation-assisted upper airway procedure. Chin Med J 2012 Mar; 125 (5): 869–73
Na HS, Song IA, Park HS, et al. Dexmedetomidine is effective for monitored anesthesia care in outpatients undergoing cataract surgery. Korean J Anesthesiol 2011 Dec; 61 (6): 453–9
Ryu JH, Lee SW, Lee JH, et al. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth 2012 Mar; 108 (3): 503–11
Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012 Mar 21; 307 (11): 1151–60
Acknowledgements and Disclosures
This article was updated from Drugs 2011: 71 (11): 1481-501,[7] and was reviewed by J.L. Ard Jr, Department of Anesthesiology, NYU Medical Center, New York, NY, US; S. Bergese, Departments of Anesthesiology and Neurological Surgery, The Ohio State University Medical Center, Columbus, OH, USA; V.R. Belum, Departments of Anesthesiology and Neurological Surgery, The Ohio State University Medical Center, Columbus, OH, USA; L. Liu, Department of Anesthesia & Perioperative Care, UC San Francisco, San Francisco, CA, USA.
The preparation of this article was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the articles. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, G.M., Hoy, S.M. & Lyseng-Williamson, K.A. Dexmedetomidine: A Guide to Its Use for Sedation in the US. Clin Drug Invest 32, 561–567 (2012). https://doi.org/10.1007/BF03261910
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261910